1. n. [Drilling Fluids]
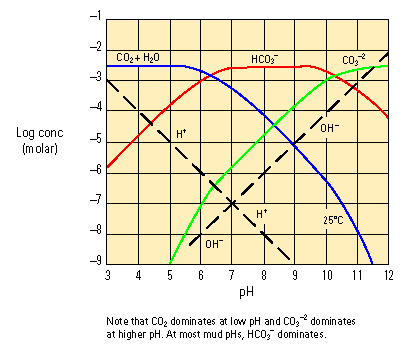
An anion with formula CO3–2. Carbonate chemistry involves a pH-dependent equilibrium between H2O, H+, OH–, CO2, HCO3– and CO3–2. At low pH, carbon dioxide [CO2] dominates. As pH rises from acidic toward neutral, HCO3– ions dominate. As pH rises above neutral, CO3–2 ions dominate. If no component is lost from the system (such as CO2 gas evolving), changing pH up and down continually reverses the relative proportion of the carbonate species. Carbonates play several important roles in water mud chemistry.
See related terms: alkalinity, buffered mud, calcium hydroxide, calcium sulfate, carbonate test, Garrett Gas Train, hydrolysis, lignite, sodium bicarbonate, sodium carbonate